Covalent Bond
The bond formed by sharing a pair of electrons between two atoms are known as Covalent Bond. Carbon forms covalent bond. Carbon exists in two forms- as free state and as combined state. Free form of carbon is found in graphite, diamond and fullerene. In combined state, carbon exists as Carbon-dioxide, Glucose, Sugar etc.
Properties of Covalent Compounds
• The covalent compounds do not exist as ions but they exist as molecules
• They exist at room temperature, as liquids or gases. However, a few compounds
also exist in the solid state e.g. urea, sugar, etc.
• The melting and boiling points of covalent compounds are generally low
• Covalent compounds are generally insoluble or less soluble in water and in other
polar solvents
• These are poor conductors of electricity in the fused or dissolved state
• Since the covalent bond is localized in between the nuclei of atoms, it is directional
in nature
• A covalent bond can be formed in different ways. When a bond is formed by mutual
sharing of one pair of electrons it is known as a 'single covalent bond', or simply 'a
single bond'. When a bond is developed due to mutual sharing of more than one
pairs of electrons it is termed as 'multiple covalent bond'. Such bonds can be a
double covalent bond or a triple covalent bond.
Formation of hydrogen molecule (H2)
Atomic Number of H = 1
Electronic configuration of H = 1
Valence electron of H = 1
Hydrogen forms a duet, to obtain stable configuration. This configuration is similar to helium (a noble gas).
Since, hydrogen has one electron in its valence shell, so it requires one more electron to form a duet. So, in the formation of hydrogen molecule; one electron from each of the hydrogen atoms is shared.
Chlorine Molecule
Chlorine atom has seven valence electrons. Thus, each Cl atom requires one more
electron to acquire the nearest noble gas configuration (Ar:2, 8, 8). This they do by
mutual sharing of one pair of electrons as shown below.
Double Bond Oxygen Molecule
An oxygen atom has six electrons in its valence shell. As a result, it requires 2 more
electrons to achieve the nearest noble gas configuration. When two oxygen atoms share
two pairs of electrons this is achieved:
Triple Bond Nitrogen Molecule
Nitrogen atom has five electrons in its valence shell. It requires three more electrons to
acquire a stable configuration of the nearest noble gas (neon). This is done by mutually
sharing three pairs of electrons as shown below.
Allotropes of Carbon
Allotropy: The phenomenon of existence of an element in different forms having
different physical properties but identical chemical properties is called allotropy and the
various forms are called allotropic forms or allotropes.
Crystalline form: Diamond, Graphite
Amorphous form: Coal, Coke, Charcoal (or wood charcoal), Animal Charcoal (or
bone black), Lamp black, Carbon black, Gas carbon and Petroleum coke.
Diamonds and graphite are two crystalline allotropes of carbon. Diamond and graphite
both are covalent crystals. But, they differ considerably in their properties.
compare the properties of diamond and graphite
Fullerenes
Fullerenes are allotropes of carbon that were discovered as recently as 1985. They have
been found to exist in the interstellar dust as well as in geological formations on earth.
They are large cage like spherical molecules with formulae C32, C50 C60, C70, C76, C84
etc. The most commonly known fullerene is C60 which is named as 'buckminster
fullerene after the designer of the geodesic dome, American architect Buckminister.
Organic Compounds – Compounds of carbon and hydrogen.
Organic Chemistry – The branch of Chemistry that deals with the study of compounds
of carbon and hydrogen.
Distinguishing features of Organic Compounds
1. Types of Linkages – Organic compounds generally contain covalent linkages while
Inorganic Compounds are ionic in nature.
2. Melting and Boiling Point – Organic Compounds have low melting and boiling
points because of their covalent nature. Inorganic Compounds usually have high
melting and boiling points.
3. Solubility – Organic Compounds are insoluble in water but soluble in organic
solvents.
4. Electrical Conductivity – Organic Compounds are bad conductors of electricity while
inorganic compounds are good conductors of electricity.
5. Nature of reactions – Organic reactions are complicated and slow whereas Inorganic
reactions are instantaneous.
6. Stability – Organic Compounds are less stable to heat than Inorganic Compounds.
7. Combustibility – Organic Compounds are combustible and generally leave no
residue, when burnt. Inorganic Compounds are incombustible.
Unsaturated Hydrocarbons
Compounds of carbon and hydrogen that contain one double covalent bond between
carbon atoms (carbon=carbon) or a triple covalent bond between carbon atoms
are called unsaturated hydrocarbons. In these molecules, since all
the bonds of carbon are not fully utilised by hydrogen atoms, more of these can be
attached to them. Thus, they undergo addition reactions (add on hydrogen) as they have
two or more hydrogen atoms less than the saturated hydrocarbons (alkanes).
Unsaturated hydrocarbons can be divided into 'alkenes' and 'alkynes' depending on the
presence of double or triple bonds respectively.
Properties of Saturated and Unsaturated Compounds
Classification of Hydrocarbons
Homologous Series
A series of carbon compounds in which same functional group substitutes the hydrogen atom is called a homologous series. These compounds have similar chemical properties due to the addition of same kind of functional group throughout the chain.
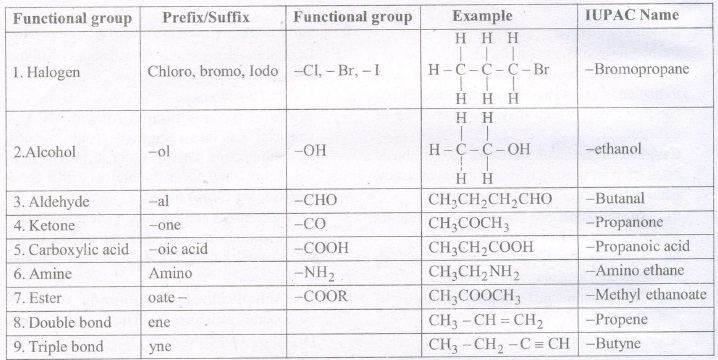
Nomenclature of Carbon Compounds
"Nomenclature is the system of assigning a proper name to a particular carbon compound on the
basis of certain rules."
Most of the carbon compounds have two types of names:
• Trivial Names
• IUPAC Names
Trivial Names
The trivial names are the commonly used names of carbon compounds. They are derived mostly
from the source of the compound e.g., the name of formic acid is derived from 'formicus' the
Greek word meaning red ants. Names arrived in this way were ambiguous and repeating.
IUPAC Names
With the large growth of carbon compounds, it was necessary to name these compounds in a
more systematic way. A committee called the 'International Union for Pure and Applied
Chemistry' (IUPAC) put forward a system of giving proper scientific names to carbon based
compounds. The names derived by their rules are the names followed all over the world and in
short are called IUPAC names.
In this system the name of a carbon compound has three main parts as mentioned below:
Wood Root
This denotes the number of carbon atoms present in a given molecule. For e.g., C1-Meth, C2-
Eth, C3 - Prop, C4- But.
Isomers and Isomerism
Compounds which have same molecular but different structural formulae are called isomers and
the phenomenon is known as isomerism.
1. Chain Isomerism – The isomerism in which the isomers differ from each other due to the
presence of different carbon chain skeletons .Example: n-butane and iso-butane.
2. Position Isomerism – the type of isomerism in which the isomers differ in the position of the
functional group. Example: But-1-ene and But-2-ene.
3. Functional Isomerism – The type of isomerism in which the isomers differ in structure due to
the presence of different functional groups.
Chemical Properties of Carbon Compounds
Most of the carbon-containing compounds associated with hydrogen i.e., hydrocarbons are fuels
that produce heat on burning. Petroleum products like natural gas, petrol, diesel, kerosene, heavy
oils etc., and in a larger sense, wood, biogas, charcoal and coke are all rich source of carbon
compounds used as fuels.
Combustion
Combustion means the burning of a substance. It is a process that is highly exothermic i.e.,
produces a lot of heat. The products of combustion of carbon and its compounds are heat energy,
carbon dioxide and water (vapour).
In order that a fuel undergoes combustion, three basic requirements are to be present.
• A combustible substance: All carbon compounds are combustible, but carbon as diamond is
not. Petrol is a combustible substance.
• A supporter of combustion: Atmospheric air or oxygen gas is a supporter of combustion. In
their absence, combustion will not be supported. Carbon dioxide or nitrogen gases do not
support combustion.
• Heating to ignition temperature: A minimum amount of temperature or heat is required to
enable a fuel to catch fire. Coal has a high ignition temperature; a matchstick cannot produce
enough heat to ignite it. However, a matchstick can ignite paper or LPG gas as it has low
ignition temperatur.
Oxidation Carbon
Carbon undergoes oxidation by combining with oxygen at higher temperature to form to oxides,
viz., carbon monoxide (CO) and carbon dioxide (CO2). Carbon monoxide is formed, when
incomplete combustion of carbon or carbon containing fuels takes place.
CO is present in automobile exhausts (when there is incomplete combustion), volcanic gases,
chimney gases etc.
Chlorination of Methane
Chlorination of methane is carried out by taking a mixture of methane and chlorine in the
sunlight or by heating to a temperature of 250o
- 300oC. If chlorine is in excess, a number of
substitution products are obtained.
Ethanol or Ethyl Alcohol
Usually the term 'alcohol' refers to ethanol. Man has been using ethanol for thousands of years
especially in the form of wine.
The structural formula of ethanol is given as follows:
Its molecular formula is CH3CH2OH or C2H5OH
• Ethanol is colourless liquid and has a pleasant odour.
• Its boiling point is 78o C and its freezing point is -114oC.
• It is soluble in water and almost all the organic solvents.
• It is highly intoxicating in nature.
• It is combustible and burns with a blue flame.
Oxidation of Ethyl Alcohol by Acidified Potassium Dichromate
Alcohols on oxidation give aldehydes. The aldehydes on further oxidation give carboxylic acids.
Uses
All these are important chemical compounds used further by chemical industries.
• Ethyl Alcohol is used as a solvent for many organic solutes, especially which are insoluble in
water.
• It is used in the preparation of perfumes.
• It is used in the manufacturing of gasohol, which is 90% mixture of petrol (gasoline) and
10% ethanol. It helps to save gasoline.
• Ethyl Alcohol is used in making tinctures and medical syrups.
• It is used in alcoholic beverages.
• It is used as a solvent for paints, varnishes, dyes etc.
• It is used in the production of many organic compounds.
Soaps & Detergents
Introduction
Soaps or detergents are cleansing agents that are capable of reacting with water to dislodge these
foreign particles from a solid surface (e.g. cloth or skin). Soaps have their origin in oils and fats
present in the animal and plant kingdom and synthetic detergents find their source in mineral oils
(hydrocarbon compounds of petroleum or coal). Chemically speaking, Soaps are sodium or
potassium salts of higher fatty acids like stearic, palmitic and oleic acids can be either saturated
or unsaturated. They contain a long hydrocarbon chain of about 10-20 carbon with one
carboxylic acid group as the functional group.
Saturated fatty acids such as stearic and palmitic etc. contain only single bonds in their molecule,
while unsaturated fatty acids such as oleic, linoleic etc., contain one or more double bonds. Thus,
soaps are usually a mixture of the sodium salts of the following acids:
• Stearic acid as sodium stearate (C17H35COONa) - saturated fatty acid; from vegetable oils
like linseed oil, soyabean oil.
• Palmitic acid as sodium palmitate (C15H31COONa) - saturated fatty acid; Palm oil, animal fat
• Oleic acid as sodium oleate (C17H33COONa) - unsaturated fatty acid; Vegetable oils like
linseed oil, soyabean oil.
When soap is made from the sodium salts of the acids of cheap oils or fats, the resulting soap is
hard. These soaps contain free alkalis and are mainly used as washing bars for laundry. When
soap is prepared from the potassium salts of the acids of good grade oils and fat, it results in soft
soap. These soaps do not contain free alkalis. They produce more lather and are used mainly as
toilet soaps, shaving cream and shampoos.
Difference between Toilet Soap and Laundry Soap
Synthetic Detergents
A synthetic detergent is any synthetic substance, other than soap, that is an effective cleanser and functions equally well as a surface-active agent in hard or soft water. It is a non-soap cleanser that exerts its effect by lowering the surface tension of an aqueous cleansing mixture.
Advantages of Detergents
• Synthetic detergents clean effectively and lather well even in hard water and salt water (sea
water). There is no scum formation.
• Since detergents are the salts of strong acids they do not decompose in acidic medium. Thus
detergents can effectively clean fabric even if the water is acidic.
• Synthetic detergents are more soluble in water than soaps.
• They have a stronger cleansing action than soaps.
• As detergents are derived from petroleum they save on natural vegetable oils, which are
important as essential cooking medium.
Disadvantages of Detergents
Detergents are surface-active agents and cause a variety of water pollution problems.
• Many detergents are resistant to the action of biological agents and thus are not
biodegradable. Their elimination from municipal wastewaters by the usual treatments is a
problem.
• They have a tendency to produce stable foams in rivers that extend over several hundred
meters of the river water. This is due to the effects of surfactants used in their preparation.
Thus they pose a danger to aquatic life.
• They tend to inhibit oxidation of organic substances present in wastewaters because they
form a sort of envelope around them.
Differences between Soaps and Detergents
If a straight chain hydrocarbon is used in the detergent instead of a branched chain hydrocarbon,
then the detergent becomes biodegradable. Thus the major disadvantage of detergents can be
overcome.